Breakthrough In Acute Pain Management: FDA Approves First-In-Class Non-Opioid
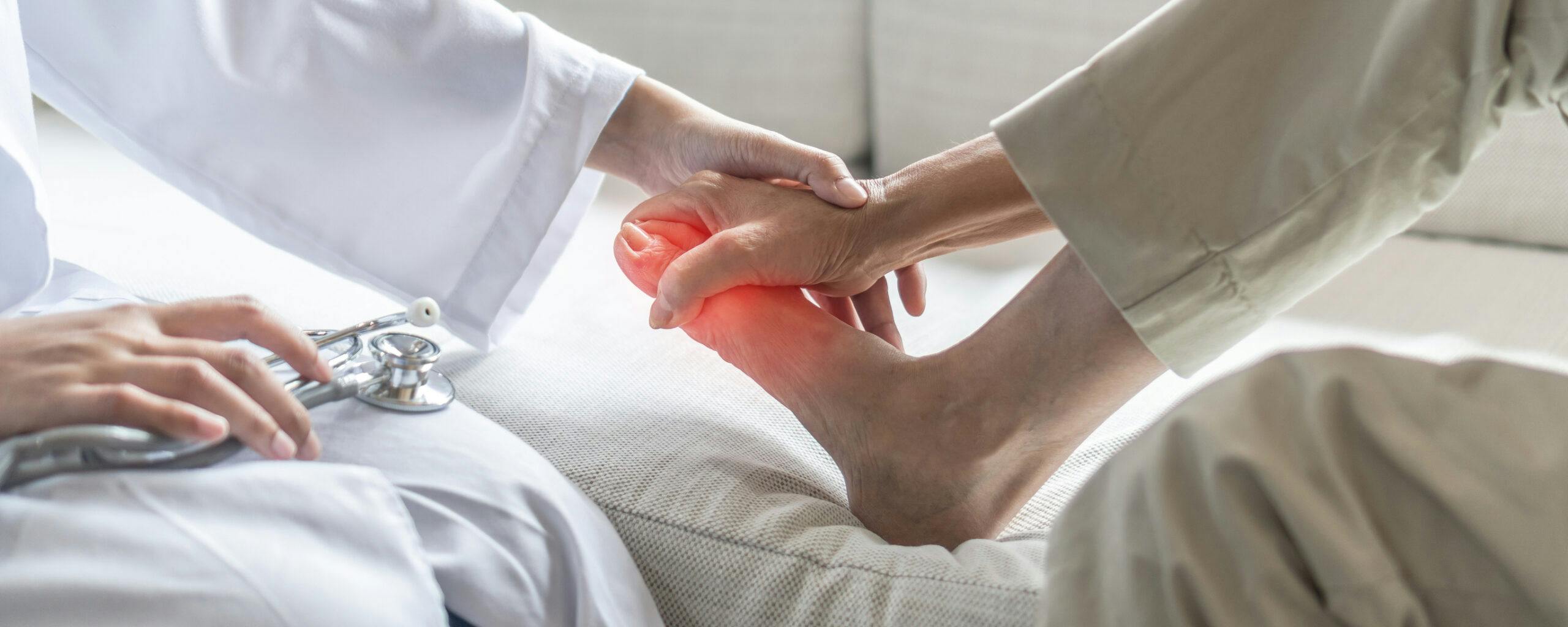
On Thursday, the U.S. Food and Drug Administration (FDA) approved Journavx (suzetrigine) 50 mg oral tablets, a first-in-class non-opioid analgesic for the treatment of moderate to severe acute pain in adults.
Live Your Best Retirement
Fun • Funds • Fitness • Freedom
Developed by Vertex Pharmaceuticals, Journavx represents a novel approach to pain management, targeting sodium channels in the peripheral nervous system to block pain signals before they reach the brain, offering an alternative to addictive opioids for post-surgical pain.
The FDA highlighted this approval as a major public health milestone, providing patients with a safer pain management option while addressing the opioid crisis. The agency has long promoted the development of non-opioid therapies through its Overdose Prevention Framework, issuing guidance to encourage innovative analgesics and supporting clinical practice guidelines for acute pain management. Journavx’s application received Breakthrough Therapy, Fast Track, and Priority Review designations, reflecting its therapeutic significance.
Efficacy was demonstrated in two randomized, double-blind, placebo- and active-controlled clinical trials involving patients recovering from abdominoplasty and bunionectomy. Both trials showed that Journavx significantly reduced pain compared to placebo, with participants allowed to use ibuprofen as “rescue” medication if needed. Safety data from 874 participants in these trials, along with supportive data from 256 patients in a separate open-label study, showed that common adverse effects included itching, muscle spasms, rash, and elevated creatine phosphokinase. Journavx is contraindicated with strong CYP3A inhibitors and should not be taken with grapefruit-containing foods or drinks.
Journavx joins the broader category of non-opioid analgesics, which includes acetaminophen, NSAIDs, COX-2 inhibitors, topical agents, and adjuvant therapies. Unlike opioids, these drugs relieve pain without binding to central opioid receptors, avoiding risks of dependence, tolerance, and respiratory depression. Journavx distinguishes itself as the first approved drug to block pain at peripheral nerves without affecting the brain, offering a promising new option for acute pain management while reducing reliance on opioid medications.
Most side effects in clinical trials were rated as mild to moderate and resolved without long-term issues. While no serious adverse events were directly linked to the drug in primary trials, the FDA and medical guides list several precautions:
- Liver Function: Patients with moderate hepatic impairment require a lower dose. The drug is not recommended for those with severe liver disease (Child-Pugh Class C).
- Kidney Function: It should be avoided in patients with severe renal impairment (eGFR <15 mL/min).
- Fertility & Pregnancy: It may temporarily reduce the chance of becoming pregnant while on treatment. There is currently no human data on its safety during pregnancy or breastfeeding.
Originally Published at Daily Wire, Daily Signal, or The Blaze
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0